L.M. Almond,a J. Hutchings,a H. Barr,a,b J. Day,c N. Stonea and C. Kendalla
aBiophotonics Research Unit, Gloucestershire Royal Hospital, Great Western Road, Gloucester, Gloucestershire GL1 3NN, UK
bDepartment of Oesophagogastric Surgery, Gloucestershire Royal Hospital, Great Western Road, Gloucester, Gloucestershire GL1 3NN, UK. E-mail: [email protected]
cInterface Analysis Centre, University of Bristol, 121 St Michaels Hill, Bristol BS2 8BS, UK
Introduction
Cancers of the stomach and oesophagus pose major global public health challenges. In order to improve outcomes for patients with these cancers, early detection and treatment of pre-cancerous lesions is essential. However, currently up to 50% of oesophageal and gastric dysplasia (pre-cancer) is macroscopically invisible and so cannot be detected by clinicians during endoscopy. As a result, upper gastrointestinal (GI) cancers are often detected at a late stage by which time they are incurable. Urgent action is required to develop a clinical test that could allow real-time in vivo diagnosis of pre-cancerous dysplasia eliminating the need for biopsy and enabling immediate endoscopic therapy to be targeted at the pre-cancerous lesion resulting in improved prognosis.
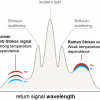
Fibre-optic Raman spectroscopy has shown great potential as a diagnostic tool in the upper GI tract, as well as at other tissue sites. Raman spectroscopy generates a unique tissue spectrum which facilitates analysis of the biochemical constituents in a tissue sample and can be used as an adjunct to histological diagnosis. The technique involves excitation of the target tissue with a monochromatic laser. The majority of the excitation laser light is elastically scattered by the tissue (the wavelength is unchanged) but a small proportion, approximately 1 in 106 photons, is inelastically scattered (Raman scattering), with an altered wavelength, the Raman shift.
Technological developments have highlighted the feasibility of fibre-optic Raman spectroscopy as an endoscopic diagnostic tool during in vivo assessment of patients. However, for Raman spectroscopy to be clinically applicable in vivo it must be rapid, cost-effective and highly accurate for pre-cancerous dysplasia. In addition, there must be a mechanism to enable probing of hollow viscera that are 15–40 cm (oesophagus) and 40–60 cm (stomach) away from the oral cavity. This presents the challenge of coupling Raman spectroscopy to a fibre-optic probe whilst maintaining low background signal, with efficient delivery and collection optics, and whilst adhering to the size specifications necessary for endoscopic use.
In this article we review the state of the art development of fibre-optic Raman probes for in vivo tissue diagnosis in the upper gastrointestinal tract. We identify the major breakthroughs in probe development, reflect on results of the first in vivo trials, and discuss the crucial challenges that must be overcome before Raman spectroscopy can be considered for widespread clinical implementation.
Why Raman spectroscopy?
Raman spectroscopy is a non-invasive, reproducible and cost-effective technique that objectively measures highly specific biochemical information within a clinically practicable timescale (seconds). Raman spectroscopy can also be used in combination with fibre-optic probes enabling assessment within the upper gastrointestinal tract and other hollow organs. In addition, the technique does not require tissue preparation (fixation or staining) prior to use, making it perfect for use as an in vivo diagnostic tool.
Fibre-optic probe development
A modern fibre-optic Raman spectroscopy system incorporates a single monochromator, a high quality charged-coupled device (CCD) detector, a series of optical filters, a computer and a specialised fibre-optic probe (Figure 1). Near infrared (NIR) (785–830 nm) excitation wavelengths are utilised to help to minimise tissue fluorescence and spectral disruption from optical components.

Raman signals are inherently weak, and biochemical changes between normal and invisible dysplastic (pre-cancerous) cells are subtle. When analysing tissue, excitation laser power must be kept low to prevent any chance of thermal injury, meaning signal intensity is limited. As a result, Raman probes must have sufficient collection efficiencies to ensure an adequate signal-to-noise ratio (SNR). In addition their design must comply with a number of dimensional specifications to ensure compatibility with medical endoscopes.
Fibre-optic Raman probes (not endoscope compatible) are well established and some are commercially available. The majority utilise separate illumination and collection fibres to reduce unwanted intrinsic fibre signal and typically include multiple 100–200 µm diameter fibres in conjunction with in-built optical filters.
In 1998, Mahadevan-Jansen et al. published in vivo results using a fibre-optic Raman probe to analyse dysplastic change in cervical tissue.1 Silica–silica fibres were shown to cause less signal disruption than sapphire and liquid guide fibres despite their tendency to generate significant background signal at low wavenumbers (below 900 cm–1). The probe consisted of a single excitation fibre and 50 collection fibres and had a total diameter of 12 mm. The excitation fibre was positioned at the edge of the probe, and the laser output (at 789 nm) was reflected by a gold mirror at an angle to produce a 900 µm diameter excitation spot on the tissue surface. A bandpass filter on the distal tip of the excitation fibre blocked fluorescence and Raman scattering generated by the fibre, but transmitted the excitation light. Although demonstrating that Raman spectra could feasibly be measured in vivo using a fibre-optic, the dimensions of this probe and the time taken for spectral acquisition (90 s) meant this design was impractical for use in the gastrointestinal tract.

The next important challenge was to develop an effective miniature fibre-optic probe that employed state-of-the-art probe technology but which was compatible for use with a medical endoscope. An endoscope is 90 cm in length and has a 2.8 mm diameter instrument channel through which a Raman probe could be delivered. This presents a considerable technical challenge; to package a probe with all its constituent parts (filters, collimating lenses, illumination and collection channels, and external casing) into a durable device with a diameter smaller than 2.8 mm!
In 1999 Shim et al. reported on utilising an endoscope compatible, fibre-optic Raman probe consisting of one central delivery fibre (400 µm diameter) surrounded by seven collection fibres each with a 300 µm diameter (Visionex, Inc., Sunnyvale, CA, USA, probe).2 Filters were placed over the fibres approximately 2.5 cm from the probe tip and the collection fibres were part bevelled so that they predominantly collected signal from a zone in front of the delivery fibre and at a specified collection depth, determined by the bevelled angle. This design provided a means of controlling the sampling volume whilst optimising collection efficiency.
In 2000, Shim et al. reported the first in vivo Raman measurement of gastrointestinal tissue using the Visionex probe described above (Table 1).3 In this feasibility study the probe was passed through an endoscope into the oesophagus, stomach and colon. Twenty patients successfully underwent the endoscopic procedure following routine sedation and no complications were reported. The probe was positioned to touch an area of mucosa for 5 s during which time readings were taken. The probed site was then biopsied to allow comparison of Raman spectra with histopathology at that site. Subtle differences were reported in spectra from normal and diseased areas but the data acquired were not sufficient to discriminate between pathological groups. Whilst demonstrating feasibility, Shim et al. concluded that more work was needed to enhance the SNR, to minimise acquisition times, and to improve data interpretation before reliable results could be generated. This study illustrated that endoscopic Raman spectroscopy could be performed safely in human patients, but was not large enough to enable comparison with results generated using laboratory based Raman spectrometers.
Table 1. In vivo Raman spectroscopy in the upper GI tract (trials are in humans unless stated). All probes incorporated miniature in-built optical fibres. +NBI—narrow band imaging, *AFI—autofluorescence imaging.
Year | Ref. | Site | Number of patients/spectra | Measurement parameters | Probe design | Accuracy/conclusions |
2000 | Oesophagus, stomach (and colon) | 20 patients | Diode laser at 785 nm | Visionex probe, 7 bevelled collection fibres, 1 excitation fibre | Feasibility demonstrated | |
2005 | Oesophagus | 65 | Diode laser at 785 nm | Single delivery fibre, 6 bevelled collection fibres Collection depth 800 µm | Sensitivity 86% and specificity 88% for dysplasia (compared to non-dysplastic oesophagus) | |
2007 | Rat oesophagus and stomach |
| Ti:sapphire laser at 785 nm | Single central delivery fibre, 8 collection fibres | Feasibility demonstrated | |
2010 | Stomach | 71 patients | Diode laser at 785 nm | Single central delivery fibre, 32 collection fibres (diameter 1.8 mm) | Delineation of malignant gastric ulcers (from benign gastric tissue): | |
2011 | Oesophagus | 27 patients | Diode laser at 785 nm | Probe as above. Multimodal imaging—NBI+ plus AFI* | Delineation of oesophageal cancer (from normal oesophageal mucosa): |
Early fibre-optic probes collected significant spectral contributions from tissue that is deeper than 500 µm. More recently a greater emphasis has been placed on targeted illumination of the epithelial lining of the gastrointestinal tract. Pre-cancerous dysplasia develops in epithelial cells and, by definition, has not yet invaded deeper structures. For optimum diagnostic accuracy, probes must collect signal from the epithelial layer and minimise spectral contributions from scattering occurring in deeper tissues.
In 2005, Wilson and co-workers published results of an in vivo trial in the upper gastrointestinal tract of humans. They used a probe consisting of a central delivery fibre surrounded by six 300 µm diameter bevelled collection fibres that achieved an estimated sampling depth of 500 µm.4 In conjunction with a diode laser (emitting at 785 nm) and a modern CCD detector they reported an accuracy of 87% for delineating between normal oesophageal tissue and dysplasia.
Huang and co-workers recently described the use of a ball lens at the distal tip of a novel Raman probe to focus excitation light onto the epithelium and help collimate scattered light into the collection fibres.5 Day et al. have used a graded index (GRIN) lens to the same effect in their novel probe that has been specifically designed for spectral measurement in the oesophagus.6 This probe consists of a single illumination and a single collection fibre with collimating lenses to ensure optimal optical coupling, and in-built filters to exclude intrinsic signal generated within the optical cables. The single fibre, which has a small aperture, shows a high degree of selectivity for detecting spectral signal arising from the epithelium. This probe was used to interrogate oesophageal biopsy tissue in vitro and demonstrated that Barrett’s oesophagus, dysplasia and cancer could be successfully separated with 71–88% sensitivity and 77–99% specificity.6
In 2010, following a number of preliminary in vitro and in vivo studies in rats, Huang and co-workers published a series of results from in vivo trials of endoscopic Raman spectroscopy for the detection of gastric neoplasia in humans. They used a 1.8 mm diameter Raman probe consisting of 32 collection fibres surrounding a single central delivery fibre. The probe had two stages of optical filters, at its proximal and distal ends, and used 785 nm excitation. Each spectrum was acquired in 0.5 s with a laser light irradiance of 1.5 W cm–2. In December 2010 they reported a diagnostic sensitivity of 82.1% and a specificity of 95.3% for delineating malignant gastric ulcers from benign tissue.7 In 2011, the same Raman probe system was used in the oesophagus demonstrating a sensitivity of 97.0% and a specificity of 95.2% for detecting oesophageal cancer.8
Conclusions
It is now eleven years since the first in vivo trial of Raman spectroscopy in the gastrointestinal tract and there has been considerable progress in probe design, NIR diode laser availability, spectrometer capability and data processing during this time. Recent studies have demonstrated the ability to collect clinically applicable results in practical timescales. However, further developments remain essential to reduce background spectral signal, optimise collection efficiency and ensure that signal is derived from the epithelial surface so that the earliest pre-cancerous changes can be detected whilst still at a potentially curable stage.
References
- A. Mahadevan-Jansen, M.F. Mitchell, N. Ramanujam, U. Utzinger and R. Richards-Kortum, “Development of a fiber optic probe to measure NIR Raman spectra of cervical tissue in vivo”, Photochem. Photobiol. 68(3), 427–31 (1998).https://doi.org/10.1111/j.1751-1097.1998.tb09703.x
- M.G. Shim, B.C. Wilson, E. Marple and M. Wach, “A study of fiber-optic probes for in vivo medical Raman spectroscopy”, Appl. Spectrosc. 53, 619–627 (1999).https://doi.org/10.1366/0003702991947225
- M.G. Shim, L.M. Song, N.E. Marcon and B.C. Wilson, “In vivo near-infrared Raman spectroscopy: demonstration of feasibility during clinical gastrointestinal endoscopy”, Photochem. Photobiol. 72(1), 146–150 (2000).
- L.M. Wong Kee Song, A. Molckovsky, K.K. Wang, L.J. Burgart, B. Dolenko, R.J. Somorjai and B.C. Wilson, “Diagnostic potential of Raman spectroscopy in Barrett’s esophagus”, Proc. SPIE 5692, 140–146 (2005).https://doi.org/10.1117/12.584986
- J. Mo, W. Zheng and Z. Huang, “Fiber-optic Raman probe couples ball lens for depth-selected Raman measurements of epithelial tissue”, Biomed. Opt. Express 1(1), 17–30 (2010).https://doi.org/10.1364/BOE.1.000017
- J.C. Day, R. Bennett, B. Smith, C. Kendall, J. Hutchings, G.M. Meaden, C. Born, S. Yu and N. Stone, “A miniature confocal Raman probe for endoscopic use”, Phys. Med. Biol. 54(23), 7077–7087 (2009).https://doi.org/10.1088/0031-9155/54/23/003
- M.S. Bergholt, W. Zheng, K. Lin, K.Y. Ho, M. Teh, K.G. Yeoh, J.B. So and Z. Huang, “Raman endoscopy for in vivo differentiation between benign and malignant ulcers in the stomach”, Analyst 135(12), 3162–3168 (2010).https://doi.org/10.1039/c0an00336k
- M.S. Bergholt, W. Zheng, K. Lin, K.Y. Ho, M. Teh, K.G. Yeoh, J.B. So and Z. Huang, “In vivo diagnosis of esophageal cancer using image-guided Raman endoscopy and biomolecular modelling”, Technol. Cancer Res. T. 10(2), 103–112 (2011).https://doi.org/10.7785/tcrt.2012.500185
- Y. Hattori, Y. Komachi, T. Asakura, T. Shimosegawa, G. Kanai, H. Tashiro and H. Sato, “In vivo Raman study of the living rat esophagus and stomach using a micro-Raman probe under an endoscope”, Appl. Spectrosc. 61(6), 579–584 (2007).https://doi.org/10.1366/000370207781269747
- C. Kendall, N. Stone, N. Shepherd, K. Geboes, B. Warren, R. Bennett and H. Barr, “Raman spectroscopy, a potential tool for the objective identification and classification of neoplasia in Barrett’s oesophagus”, J. Pathol. 200(5), 602–609 (2003).https://doi.org/10.1002/path.1376