Maxime Boulet-Audet and Chris Holland
Department of Zoology, University of Oxford, South Parks Road, Oxford OX1 3PS, UK
Introduction
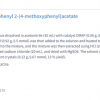
Plant toxins are commonly used by many invertebrates, as well as a few vertebrates, as an effective defence against predation. However, such behaviour has recently been observed in a mammal.1 The African crested rat, Lophiomys imhausi, applies a potent toxin to the fur alongside its flanks. It acquires this toxin from Acokanthera schimperi,1 a shrub abundant in East Africa and dubbed the “poison arrow tree” due to its use by local hunters. The poison itself, a cardenolide called ouabain,2 appears not to affect the rat but causes pain and cardiac dysrhythmia to most vertebrates and even a small amount can kill an elephant. However, in the hands of a skilled medic, this cardiac glycoside is a well known treatment for congestive heart failure.3
To obtain this poison, the African crested rat has been observed gnawing and chewing the bark of the Acokanthera to release ouabain, which is water soluble due to its hydroxyl groups.1 At the same time, the rat secretes proteins in its saliva that can bind plant polyphenols and precipitate them.4 Once the plant material has been thoroughly chewed, the coarse colloid mixture is applied by the rat onto its two lateral fur lines, extending from behind the ears across its flank.1 These fur strips consist of highly specialised hairs, normally hidden beneath longer hairs to prevent exposure to rain and sun, which allows the spittle to be rapidly wicked-up and stored.1
When threatened, Lophiomys uses its specialised dermal muscles to erect the hairs covering the poisonous lines, forming an easily identifiable dorsal crest.1 The rat then directs the attention of the predator toward this lateral fur line in a taunting display. Any predator that bites this area exposes itself to the poison, as evidenced by numerous anecdotes of dogs dying from tackling this rat. As part of the defence mechanism of the rodent, we investigated its highly specialised hairs using infrared spectroscopy as well as gravimetry.
Methods
Lophiomys imhausi lateral hairs were taken from a skin belonging to the National Museums of Kenya (Catalogue number NMK 180396 Coll Sept. 2010 Field No D. 1). The saliva extract from the samples was prepared by washing several hairs with demineralised water and filtered using 0.2 mm pore size membrane under vacuum. The spectra acquisition was performed using a Nicolet 6700 Fourier transform infrared (FT-IR) spectrometer (Thermo Scientific, Madison, WI, USA) equipped with a liquid–nitrogen-cooled mercury–cadmium–telluride MCT-A detector together with a single bounce diamond attenuated total reflection (ATR) accessory (Golden Gate, Specac, UK). This sampling method allowed the selective probing of the first few microns of the hair’s surface. Spectra were obtained from an average of 64 scans at 4 cm–1 resolution. All spectral operations were performed using Omnic 7.3 (Thermo Scientific, Madison, WI, USA). A simple ATR correction was applied to compensate for the penetration depth dependency on the wavelength. The spectra were offset with the 1900–1850 cm–1 region before normalisation with the integral of the 1700–1300 cm–1 region. The spectra were recorded from different parts of a rat hair before washing with demineralised water for comparison. The wicking properties of the hair were measured with a Q500 thermal gravimetric analyser (TA Instruments) by immersing the first 1 mm of the tip of the hair in demineralised water. The mass of the suspended hair was recorded as a function of time whilst the water was absorbed by capillarity up the hair.
Results and discussion
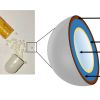
As shown in Figure 1, the specialised hairs are hollow, similar to those of some arctic mammals to aid with insulation but, for the rat, they are also perforated, thus suggesting a totally different function. These hairs consist of a mesh cylinder of 200–250 µm in diameter, encapsulating many fine strands of around 10 µm. We propose that this open lattice enables capillary uptake and ensures a high storage capacity for the poison payload as well as any future contact resulting in rapid release, especially if pressed flat (i.e. chewed).

As illustrated in Figure 1, a straightforward way of monitoring the capillarity effect is to record the increase in mass of the hair whilst its tip is immersed in water. We found that soon after immersion, the mass increased linearly with time at a rate of water uptake of 0.5 nL s–1 (~30 µm s–1) before saturating after 15 minutes. The ability for water to spread throughout suggests a highly effective means of uptake, regardless of where the rat applies its saliva. Furthermore, the mass of the filled hair reached a plateau at 0.42 mg, more than three times its dry weight. By assuming a hair density of 1.30 for keratin,5 the porosity of the structure can be estimated to 68%. To locate the presence of the toxin, the local chemical composition, every 2 mm along a rat hair, was probed by infrared spectroscopy using ATR.

Figure 2 illustrates that every spectrum shows protein amide I, II and III absorption bands due to α-keratin (at 1635 cm–1, 1531 cm–1 and 1320 cm–1, respectively). The amide I band shape and frequency for the maximum absorption is typical of a secondary structure dominated by α-helix structures, which is generally observed for hair.6 One note though, is the slightly lower frequency of the amide I band compared to the literature due to the anomalous dispersion of the refractive index when using a diamond internal reflection element.7
In addition, we found the infrared spectra obtained are highly dependent on the section probed. The main differences are the bands at 1018 cm–1 assigned to C–O stretching, the 1395 cm–1 peak assigned to ester groups and 1600 cm–1 associated with the OH bending. These components must come from the polyphenol compounds and the ouabain toxin as they have numerous hydroxyl functions. Since the bark and roots of Acokanthera contain vast amounts of these active ingredients, their presence in the rat’s hairs corroborates the visual observation of the animal applying the chewed bark mixture onto its fur. We observed that the spectral bands are particularly strong in the middle section of the hair, but become much weaker at the tip and root. Underneath the epidermis of the animal, the root of the hair presents no traces of these compounds and only bands characteristic of keratin proteins which is not surprising, given the unique porous structure is also absent.
When washed with water, the C–O band at 1018 cm–1 disappeared, including in the middle section (black-coloured spectrum), confirming the solubility of the compounds within. Detecting the polyphenol and ouabain by ATR/FT-IR spectroscopy proves that they are also present on the hair’s surface. By pressing a loaded hair against the ATR crystal and removing it, we observed a trace on the surface detectable by IR spectroscopy (olive-coloured spectrum). This demonstrates the ability of these porous hairs to unload their content upon pressure or contact with water, confirming our hypothesis for the mechanisms of release. As a result, both the force of the bite and the saliva of the predator release toxin from these hairs.
Conclusions
We examined the form and function of specialised hairs from the African crested rat, Lophiomys imhausi, using a combination of gravimetry and FT-IR spectroscopy. The study of the capillarity effect by gravimetry demonstrated the ability of the hair to absorb liquid from the tip to the root within minutes. Our measurements not only corroborated our visual observation of the phenomenon, but allowed the estimation of the structure’s porosity. To detect the presence of the ouabain toxin mixture in these pores, infrared spectroscopy was employed. Using spectral markers associated with polyphenols, this technique revealed the presence of Acokanthera toxic mixture in the hair with location dependant concentration. In addition, taking advantage of ATR, the spittle’s capability to be released upon contact was demonstrated.
We also confirmed that this toxic mixture can be dissolved in water. As the poison must be present in the mouth of the Lophiomys during chewing, the origin of the rodent’s immunity might be due to a mutation in the ouabain binding site affecting its saliva or digestive tract,8 but this remains unknown. The understanding of this tolerance to the cardenolide could lead to the development of a new treatment for human cardiac conditions, exploiting the metabolic pathway of the drug. Hence, this rat has demonstrated that the only limits to what we can learn from nature are the strengths of our analytical tools and techniques.
References
- J. Kingdon, B. Agwanda, M. Kinnaird, T. O'Brien, C. Holland, T. Gheysens and F. Vollrath, “A poisonous surprise under the coat of the African crested rat”, Proceedings of the Royal Society B-Biological Sciences (2011). doi: 10.1098/rspb.2011.1169
- M. Arnaud, Comptes rendus de l'Académie des Sciences 107, 1011 (1888).
- W.A. Jacobs and N.M. Bigelow, “Ouabain or g-strophanthin”, J. Biol. Chem. 96, 647–658 (1932).
- J. Muenzer, C. Bildstein, M. Gleason and DM. Carlson, “Properties of proline-rich proteins from parotid glands of isoproterenol-treated rats”, J. Biol. Chem. 254, 5629–5634 (1979).
- P. Mason, “Density and structure of alpha-keratin”, Nature 197, 179–180 (1963). doi: 10.1038/197179a0
- E.G. Bendit, “Infrared absorption spectrum of keratin. I. Spectra of α-, β- and supercontracted keratin”, Biopolymers 4, 539–559 (1966). doi: 10.1002/bip.1966.360040506
- M. Boulet-Audet, T. Buffeteau, S. Boudreault, N. Daugey and M. Pézolet, “Quantitative determination of band distortions in diamond attenuated total reflectance infrared spectra”, J. Phys. Chem. B 114, 8255–8261 (2010). doi: 10.1021/jp101763y
- E. Labeyrie and S. Dobler. “Molecular adaptation of Chrysochus leaf beetles to toxic compounds in their food plants”, Mol. Biol. Evol. 21, 218–221 (2004). doi: 10.1093/molbev/msg240