An increase in intracranial pressure (ICP) is a dangerous condition that can be caused by brain bleeds, a brain tumour, cerebral oedema, traumatic brain injury and hydrocephalus. ICP monitoring is thus a key aspect of patient care in patients with these disorders. Additionally, ICP measurements are relevant when estimating cerebral perfusion pressure (CPP), an indicator of cerebral autoregulation (CA). CPP is linked to neuronal function and neurovascular coupling, and CA defines how the brain maintains a constant blood flow. Given these broad implications and applications in clinical decision-making, precise ICP monitoring is a vital patient management tool. While current tools for ICP monitoring are precise, they can cause haemorrhage or infections and are time-consuming. Although non-invasive alternatives exist, they have limitations such as poor generalisability, low predictive capacity and a lack of reliability. Diffuse correlation spectroscopy (DCS) and near infrared (NIR) spectroscopy are emerging as promising non-invasive solutions. Notably, NIR spectroscopy has several advantages over other non-invasive methods—low cost, bedside compatibility for long-term and continuous monitoring, along with user independence.
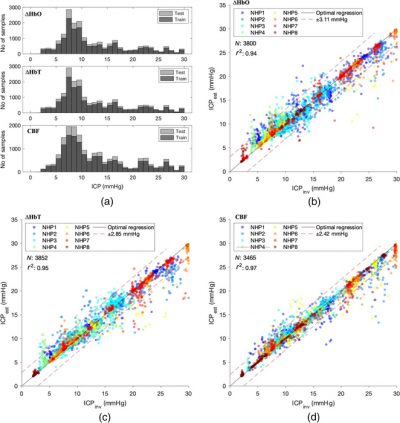
Researchers at Carnegie Mellon University (CMU) have successfully deployed a NIR spectroscopy device to continuously monitor haemoglobin concentration changes. The team built on previous research in which they estimated ICP from cardiac waveform features measured using DCS, and also identified the correlation between relative changes in oxyhaemoglobin concentration and ICP. To measure ICP using the NIR spectroscopy data, they developed and trained a random forest (RF) regression algorithm to correlate the morphology of cardiac pulse waveforms obtained through NIR spectroscopy with intracranial pressure.
To validate their algorithm, they conducted preliminary tests in a preclinical model. They measured fluctuations in invasive ICP and arterial blood pressure while profiling the changes in haemoglobin concentrations. Following this, they examined the performance of signals derived from the haemoglobin concentration and CBF to accurately verify the precision of their algorithm.
From a proof-of-concept standpoint, the results were very promising. There was a high correlation between the ICP estimated using the RF algorithm and the actual ICP measured using invasive techniques. “We showed, by validating the findings with invasive ICP data, that the trained RF algorithm applied to NIR spectroscopy based cardiac waveforms can be used to estimate ICP with a high degree of precision”, explains Jana Kainerstorfer, Associate Professor of Biomedical Engineering at CMU. Furthermore, the results indicated that the RF algorithm could interpret waveform features extracted from both NIR spectroscopy and DCS, highlighting its useability.
The parameters used in the algorithm can be obtained from NIR spectroscopy measurements, combined with electrocardiograms and mean arterial blood pressure, which are regularly used for clinical evaluation. Thus, if this RF-based platform can produce robust ICP measurements in subsequent human trials, its potential for clinical use would be tremendous.







