The 2012 Nobel prize-winning discovery that ordinary cells could be coaxed to revert to their earliest pluripotent stage ushered in the era of ethical stem cell research. Suddenly, scientists can have an inexhaustible supply of pluripotent stem cells—the most versatile of stem cells—that can become any type of cell much like how embryonic stem cells function but without the ethical troubles that hampered research in the past.
These reprogrammed cells called induced pluripotent stem cells, or iPS cells, hold great promise for regenerative medicine, where they can be used to develop tissue or organ replacement-based treatments for life-threatening diseases.
Artificially inducing ordinary cells to reset back to pluripotency, however, is a lengthy and delicate process. Obtaining iPS cells depends on chance. And knowing all they can about the complex chemical changes happening inside during reprogramming can help scientists improve the odds of attaining viable iPS cells for clinical applications. Current methods that track reprogramming status, however, use destructive and costly techniques.
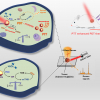
A study led by Dr Tomonobu Watanabe, professor at Hiroshima University’s Research Institute for Radiation Biology and Medicine, has shown how Raman spectroscopy could be a low-cost, simpler and non-intrusive technique in monitoring what goes on inside the cell as it transitions.
“The quality evaluation and sorting of existing cells have been carried out by investigating the presence or absence of expression of surface marker genes. However, since this method requires a fluorescent antibody, it is expensive and causes a problem of bringing the antibody into the cells”, Watanabe said.
“Solution of these problems can accelerate the spread of safe and low-cost regenerative medicine using artificial tissues. Through our method, we provide a technique for evaluating and sorting the quality of iPS cells inexpensively and safely, based on scattering spectroscopy”, he added.
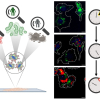
The team used Raman spectroscopy to obtain the “chemical fingerprints” of mouse embryonic stem cells, the neuronal cells they specialised into and the iPS cells formed from those neuronal cells. These data were then used to teach an AI model so it can track if the reprogramming is progressing without a hitch and verify iPS cell quality by checking for a “fingerprint” match with the embryonic stem cell.
To measure the progress, they assigned the “chemical fingerprint” of neuronal cells as the transformation starting point and the embryonic stem cell’s patterns as the desired end goal. Along the axis, they used “fingerprint” samples collected on days 5, 10 and 20 of the neuronal cells’ reprogramming as reference points on how the process is advancing.
“The Raman scattering spectrum contains comprehensive information on molecular vibrations, and the amount of information may be sufficient to define cells. If so, unlike gene profiling, it allows for a more expressive definition of cell function,” Watanabe said.
“We aim to study stem cells from a different perspective than traditional life sciences.”