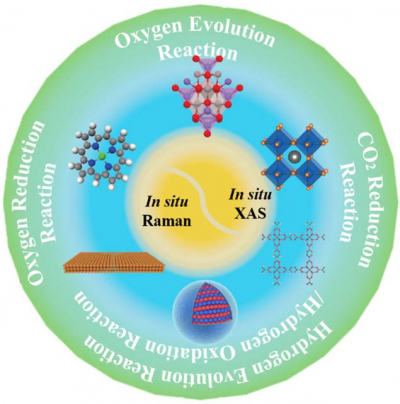
Many advanced technologies for clean energy production and conversion have been developed, including fuel cells, metal–air batteries and clean hydrogen energy produced via water splitting etc. Among these technologies, the electrochemical reduction and evolution of oxygen are two critical reactions, which almost determine the performance and efficiency of these clean-energy devices due to their sluggish reaction kinetics and large overpotential. Apart from oxygen reduction/evolution reaction (ORR/OER), the importance of the hydrogen evolution/oxidation reaction (HER/HOR) cannot be ignored either, as hydrogen can work as a powerful fuel with water as the only product. Recently, the conversion of CO2 to value-added carbon-based materials, such as ethylene, ethanol and acetic acid, can not only enrich the source of clean fuels but also artificially close the carbon cycle, making the CO2 reduction reaction (CO2RR) another important electrochemical reaction.
All the above electrocatalytic reactions occur at solid–liquid or solid–liquid–gas interfaces, and catalysts are usually used to promote the reaction by reducing the overpotential or changing the reaction pathways. Thus, the core of accelerating the interfacial electrochemical reaction is the exploration of efficient and robust electrochemical catalysts, which relies on the in-depth understanding of the reaction mechanism and intermediate process, especially at the molecular or even atomic level. These requirements for a fundamental understanding are encouraging the development of in situ or even operando characterisation techniques, including in situ Raman spectroscopy, infrared spectroscopy (IR), X-ray absorption spectroscopy (XAS) etc., that can track the dynamic catalytic processes, capture the short-lived intermediates and reveal reactive sites.
Recently, a research team led by Prof. Jian-Feng Li from Xiamen University, China, reviewed the recent applications of in situ Raman spectroscopy and X-ray absorption spectroscopy (XAS) in various energy-related reactions including ORR, OER, HER/HOR and CO2RR occurring on nano- and single-atom catalysts. Using these techniques, the oxygen-containing intermediate species were captured during the reduction of oxygen and the oxidation of hydrogen, and the structural transformation of catalysts with the change of potentials was detected during the evolution of oxygen and reduction of CO2. They also discussed the challenges and outlook in developing in situ spectroscopic technologies for deepening the fundamental understanding of these energy-related electrocatalytic processes.