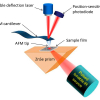
Andrew P. Dean, Jon K. Pittman and David C. Sigee
Faculty of Life Sciences, University of Manchester, Oxford Road, Manchester, M13 9PT, UK. E-mail: [email protected]
Introduction
Algae are vital to the health and wellbeing of our planet. They fix carbon through photosynthesis and form the basis of the food chain in lakes and oceans and range from giant seaweeds to tiny free-floating single cells. These free floating algae are known as phytoplankton from the Greek words phyton (plant) and planktos (wander or drift) and consists of individual cells, multicellular filaments and colonies.
At the University of Manchester we have been using Fourier transform infrared (FT-IR) spectroscopy to increase our understanding of the physiology and biotech potential of the phytoplankton. Our studies include laboratory studies, looking at algal monocultures, to field-based studies looking at algae within their natural environment, and we are particularly interested in using FT-IR spectroscopy to study the factors influencing how phytoplankton allocate carbon into lipids, carbohydrates and proteins. The way in which a cell allocates carbon to each of these groups is dependent on a number of factors such as growth rate, light intensity and nutrient availability. For instance, proteins are linked to basic functions of biosynthesis and cell division, while lipids and carbohydrates serve as intracellular reserves of carbon and energy. However, under conditions of low nutrients cells allocate more carbon to storage products such as lipids and carbohydrates, which the cell then utilises for renewed cell division when favourable conditions return. This article presents two aspects of our work in which we apply FT-IR spectroscopy to the study of the phytoplankton: (1) the use of FT-IR spectroscopy as a high-throughput technique for the rapid assessment of algal cultures for lipid content, and (2) the assessment of seasonal carbon allocation changes in selected phytoplankton species (Pedisatrum duplex, Ceratium hirundinella and Anabaena flos-aquae) within a eutrophic lake.
Laboratory studies in algal biofuels
An aspect of our work at the University of Manchester is the development of algal biofuels. During this research we regularly use FT-IR spectroscopy to study carbon allocation in monocultures of a range of algal species, and of single species across a range of growth conditions.1 The potential of algae as a source of renewable energy has received considerable attention. However, the viability of algae-derived biofuels depends on a number of factors, including the identification of species with high lipid composition, and understanding the environmental conditions that maximise lipid production. We utilise a number of techniques for the understanding of lipid synthesis in algal cells, including gene expression, genetic manipulation and gas chromatography/liquid chromatography-mass spectrometry (GC/LC-MS). Throughout this work we supplement application of these techniques with the use of FT-IR spectroscopy as a high-throughput, rapid screening tool to assess the lipid content of algal cells. For this aspect of our work we largely concentrate on the small green algae Chlamydomonas reinhardtii which is a well characterised and studied model species that is ideal for gaining an understanding of cell function and physiology. Following each experiment small volumes (<0.5 mL) of up to 96 samples of algal cells are harvested, centrifuged, re-suspended in a small volume of H2O and ~20 µL deposited onto a silicon plate. The spectra are then analysed using FT-IR spectroscopy and the resulting spectra processed for the assessment of cellular lipids. The samples may consist of a number of different cell lines, in which we wish to compare lipid composition, or may consist of a single cell line that has been subject to range of environmental conditions known to induce lipid synthesis. One such condition is nutrient deficiency, in which cells deficient in nitrogen or phosphorus cease cell division and instead store their carbon as reserves of lipid and carbohydrates (starch). Figure 1 compares cells grown in nitrogen (N) replete and nitrogen deficient media, clearly showing how the low-N conditions cause an increase in lipid synthesis that can be visualised using a lipid-specific fluorescent dye (Nile Red) that allows intracellular lipid bodies to be clearly seen. This increase in cellular lipids and carbohydrates is evident in the accompanying FT-IR spectra, in which the bands at 1740 cm–1, associated with the n(C=O) of ester groups, and the bands at 3000–2800 cm–1, from n(C–H) of saturated CH, both due primarily from lipids, are elevated under nutrient limiting conditions. In addition the carbohydrate bands at 1200–900 cm–1 [n(C–O–C) of polysaccchrides] are also highly elevated when compared to cells grown in nutrient replete media. Bands attributed to n(C=O) stretching of amides from proteins (amide I, ~1655 cm–1) and the d(N–H) bending mode of amides from proteins (amide II, ~1545 cm–1) can also be clearly seen.

FT-IR spectroscopy thus allows interesting cell lines with high lipids content, or conditions that induce lipid synthesis, to be rapidly identified for further studies using other techniques, such as LC-MS and gene expression studies. FT-IR spectroscopy is an excellent tool for these analyses as it is rapid, simple to perform, can be carried out on very small quantities of algae and the spectra provide other valuable information additional to that of lipids. It therefore has a number of advantages over traditional lipid gravimetric techniques which can be time consuming, require complex laboratory equipment, large volumes of algae and only provide information on lipids. With the increased interest in algal bioproducts the use of FT-IR spectroscopy for routine assessment of the chemical composition of algal cells is likely to grow in importance.
Environmental studies
Most FT-IR spectroscopic studies on phytoplankton have been carried out on laboratory-cultured material, with few analyses of environmental organisms. However, the high spatial resolution of the FT-IR microspectroscopy technique means that particular species can be selectively analysed within mixed environmental populations allowing us to investigate the very small algal cells and colonies, many of which are less than 20 µm in diameter. This ability to identify and study single cells/colonies facilitates the study of intra-specific hererogeneity of carbon allocation within single samples, for example, a previous study comparing Anabaena and Aphanizomenon from different depths within the lake water column showed that cells from deep in the water column exhibited reduced carbohydrates.2 It also facilitates the study of inter-species differences showing, for example, that that two co-dominant algal species (Microcystis and Ceratium) within a eutrophic lake had very different patterns of carbon (C) allocation.3 It also facilitates the analysis of temporal changes in C allocation, within single species, over an extended time period4 as is described here. This gives FT-IR spectroscopy a great advantage over conventional bulk analysis techniques for which the large volumes of algae required necessitate the bulking together of mixed-species populations—this means that individual cells/colonies cannot be analysed, and any inter- and intra-specific differences are averaged over the whole algal sample.
In order to analyse temporal changes in selected phytoplankton species we collected samples of phytoplankton over a two-year period from Rostherne Mere, a lake situated on the Cheshire plain in the UK. Cells were dried onto a reflectance slide, picked out using a light microscope and analysed using FT-IR spectroscopy. Cells of Ceratium, colonies of Pediastrum and filaments of Anabaena (Figure 2) were observed in both years with the resulting FT-IR spectra clearing showing peaks at wavenumbers corresponding to proteins, carbohydrates and lipids (Figure 3), with the absorbance of each proportional to its cellular concentration.


Pediastrum occurs as plate-like colonies, made up of a number of individual cells. Although it was only present in low numbers within the lake it had a very interesting pattern of C allocation. When Pediastrum first appeared in the water column it had very high lipid and carbohydrate reserves, but on other sampling dates these reserves were exhausted. Anabaena occurs as long filaments of individual, spherical cells. Dominant populations of Anabaena were observed in 2007 and were analysed during the population rise (to a maximum of ~800 filaments per mL) and subsequent fall. Spectra were characterised by the absence of a lipid band, and a prominent carbohydrate band during the early stages of the population increase. However, these high carbohydrate levels were progressively depleted as the population declined. Ceratium has a very characteristic appearance, looking a bit like a three-legged Eiffel tower, and was a dominant member of the phytoplankton during 2006. In contrast to the other species, Ceratium did not show any seasonal change, although the FT-IR spectroscopic analysis did identify very high levels of heterogeneity amongst the population.
It is species-specific differences, such as these that are masked when traditional bulk analyses methods are used, that emphasises the utility of FT-IR spectroscopy for these environmental studies. Such an insight into how carbon allocation varies with population development and environmental conditions has great potential for understanding the factors regulating the rise and fall of algal populations in aquatic environments, including nuisance algal blooms which are a growing problem worldwide. Through these studies, FT-IR spectroscopy has demonstrated the occurrence of large temporal and spatial differences in molecular composition both between and within species. These differences are probably related both to intrinsic differences in the molecular composition of individual species, as well as differences in algal response to changing environmental conditions. The ability of FT-IR spectroscopy to analyse single cells, colonies and species within mixed samples has considerable potential for helping us to understand these factors and has great potential for future ecological studies.
Summary
The uses of FT-IR spectroscopy described here show its utility in algal research, however, these are by no means the only applications.5 For example, other groups have used FT-IR spectroscopy for real time analysis of changing carbon patterns in live cells, 2D mapping of cells to assess intercellular spatial variation in C storage and the use of FT-IR spectroscopy for discrimination of different species. The overall conclusion from these studies is that FT-IR spectroscopy has now become a key experimental tool for algal research, both in the laboratory and in environmental studies and will continue to be so in the future.
Acknowledgements
We would like to thank James Nicholson and Beatriz Estrada for their contribution to the research on which this article is based. Thanks also to the Leverhulme Trust for funding the work carried out in this study (Grant number F/00 120/AO) and to the STFC for funding and allocation of beamtime at Daresbury Laboratory. Thanks also go to Natural England for permission to carry out sampling on Rostherne Mere and provision of boating facilities.
References
- A.P. Dean, D.C. Sigee, B. Estrada and J.K. Pittman, “Using FTIR spectroscopy for rapid determination of lipid accumulation in response to nitrogen limitation in freshwater microalgae”, Bioresource Technol. 101, 4499–4507 (2010). doi: http://dx.doi.org/10.1016/j.biortech.2010.01.065
- A.P. Dean and D.C. Sigee, “Molecular heterogeneity in Aphanizomenon flos-aquae and Anabaena flos-aquae (Cyanophyta): a synchrotron-based Fourier-transform infrared study of lake micro-populations”, Eur. J. Phycol. 41, 201–212 (2006). doi: http://dx.doi.org/10.1080/09670260600645907
- A.P. Dean, M.C. Martin and D.C. Sigee, “Resolution of co-dominant phytoplankton species in a eutrophic lake using synchrotron-based Fourier transform infrared spectroscopy”, Phycologia 46, 151–159 (2007). doi: http://dx.doi.org/10.2216/06-27.1
- A.P. Dean, M.J. Nicholson and D.C. Sigee, “Changing patterns of carbon allocation in lake phytoplankton: an FTIR analysis”, Hydrobiologia 684, 109–127 (2012). doi: http://dx.doi.org/10.1007/s10750-011-0973-0
- J.N. Murdock and D.L. Wetzel, “FT-IR microspectroscopy enhances biological and ecological analysis of algae”, Appl. Spectrosc. Rev. 44, 335–361 (2009). doi: http://dx.doi.org/10.1080/05704920902907440