Frank J.M. Rutten,* Jasim M.S. Jamur and Paul Roach
School of Pharmacy and Research Institute for Science and Technology in Medicine, Keele University, Keele ST5 5BG, United Kingdom. E-mail: [email protected]
DOI: https://doi.org/10.1255/sew.2015.a2
© 2022 The Authors
Introduction
Surfaces play a crucial role in a wide range of applications, hence have been the subject of intense study since the very early days of chemical analysis. With increasing knowledge and manufacturing capabilities, the need for ever more detailed surface analytical information has grown rapidly over the years. Whilst vibrational spectroscopies, in particular, have yielded crucial information in a wide range of settings, the very nature of these techniques does limit the information available. Mass spectrometry, on the other hand, has the capability to yield exquisitely detailed datasets but has traditionally been significantly less convenient in actual applications.
After initial use, predominantly for inorganic and materials science applications and with Alfred Benninghoven (Germany) and David Briggs (UK) among the main protagonists, advances in liquid metal ion guns and time-of-flight analysers enabled secondary ion mass spectrometry (SIMS) to become a powerful technique for the analysis of organic materials. A step-change in sensitivity revolutionised the use of SIMS in the latter part of the last century and allowed researchers to acquire detailed mass spectra from damage-prone organic materials without significantly altering the surface chemistry under investigation. Moreover, surface mass spectra containing information on large molecules can be generated from very small surface areas, especially since the introduction of cluster primary ion sources, allowing micron-scale image generation with an enormous wealth of information, in the form of a high mass-range and -resolution spectrum for each image pixel. These developments opened up entire new fields of analysis and time-of-flight SIMS (ToF-SIMS) has since been applied highly successfully in a seemingly endless range of applications from medical implants and cell biology to astrochemistry, art history and archaeology.1
Whilst much exquisite research has been and continues to be published using SIMS, a crucial restriction is the requirement for samples to be in a vacuum environment. This necessitates samples sensitive to vacuum to be especially prepared to negate any adverse effects. Moreover, sample throughput is restricted by pump-down times and sample size and geometry are limited to fit within vacuum chambers. Hence there is an important niche for an alternative MS-based surface analytical approach which can interrogate samples in an ambient environment.
Plasma-assisted desorption/ionisation mass spectrometry, applications examples
With the introduction of desorption electrospray ionisation mass spectrometry (DESI) for ambient surface analysis in a seminal paper by Cooks and group (Purdue University, USA) in Science in 2004,2 a way forward to achieve this long-held goal became apparent. This paper rightly created much excitement, and with colleagues, then at the University of Nottingham in the UK, we were keen to exploit this. In the process we developed a means to use a low-energy plasma pencil previously designed for biomedical applications, to generate mass spectra from a range of materials. Plasma-assisted desorption/ionisation mass spectrometry, PADI, see Figure 1 for the most basic setup, has since been used to investigate a range of analytes from small pharmaceutical molecules3 (Figure 2 displays analysis of tablets) to polymers.4 Amongst the plasma-based ambient MS techniques since developed (see, for example, Reference 5), PADI, which uses a very low power plasma, is characterised by a very simple and robust design, is relatively insensitive to sample geometry and can rapidly yield diagnostic mass spectra from a wide range of materials, of which a few examples are highlighted below.
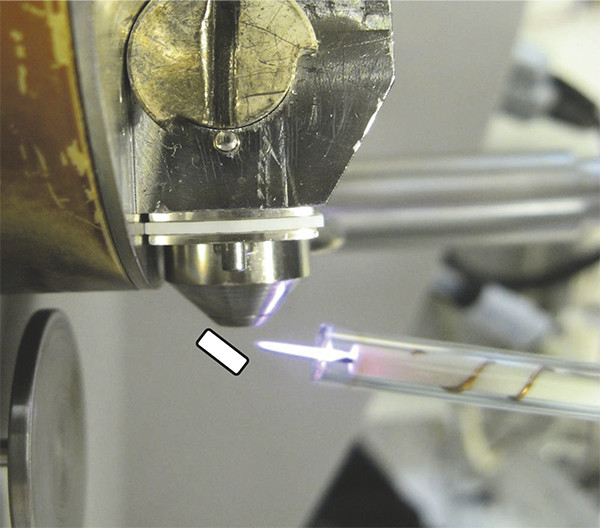
Figure 1. PADI setup. The low temperature helium RF plasma flame in front of Waters ZQ mass spectrometer inlet. In this most basic arrangement samples can be held in front of the flame with tweezers as schematically indicated.

Figure 2. PADI set-up. The plasma flame interacts with a paracetamol tablet and resultant analyte ions are transported into the Waters ZQ single quadrupole mass spectrometer through a 1⁄8” stainless steel sniffer tube.
In our initial work3 we investigated a range of over-the-counter pharmaceutical products and found that diagnostic spectra could readily be generated in positive- and negative-ion mode, including from semi-solids. Several natural products were also investigated (see Figure 3) and we were able to clearly identify nicotine in dried tobacco leaves, allicin, the predominant thiosulfate in freshly cut garlic and, more challenging, the readily decomposing lachrimator propanethial-S-oxide from freshly cut onion, which is not trivial by conventional MS.
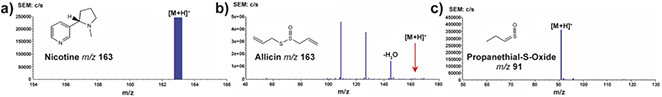
Figure 3. PADI spectra of natural products. Key: a) dried tobacco leaf; b) freshly cut garlic clove; c) freshly cut onion. Reprinted with permission from Reference 3. Copyright 2007 American Chemical Society.
In a subsequent detailed study at the National Physical Laboratory in the UK, Tara Salter and colleagues compared DESI with PADI using personal care products on skin mimics. It was found that a very quick five-second PADI spectrum was sufficient for identification of a wider range of molecules than could be analysed by DESI. Whereas they found that the PADI plasma could cause damage to the rather fragile layer of human dermal fibroblasts, this was at doses well above what was required for high quality diagnostic spectra.6
When a higher power is supplied to the radiofrequency (RF) plasma needle, the same ionisation source, instead of “merely” desorbing and ionising small molecules, can be used to investigate polymers through the generation of diagnostic ionised fragments. McKay and co-workers, in a comparative study of low temperature atmospheric plasma sources, found that PADI could be used to clearly identify PTFE [poly(tetrafluoroethylene)] (see Figure 4) and PMMA [poly(methyl methacrylate)] and that its characteristics, whilst releasing fragments indicative of the polymer repeat unit, yielded less fragmentation and hence cleaner and more readily interpreted spectra compared to the other plasma sources investigated.7
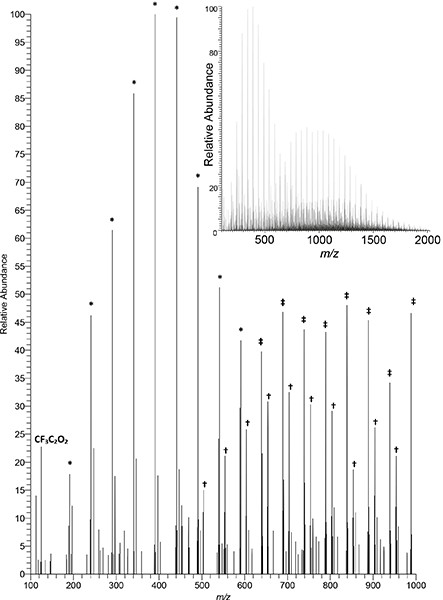
Figure 4. Negative-ion PADI spectrum of PTFE recorded on a Thermo Orbitrap mass spectrometer (unpublished data). Plasma power: 10 W. Main image displays 100–1000 m/z, insert displays full measurement range of 100–2000 m/z. Full intensity scale corresponds to 3.55 × 103 counts. Some diagnostic repeat patterns denoted by symbols: * C2O3(CF2)nF; † HNC2O6(CF2)nF; ‡ C2O4(CF2)nH.
Our current work has a dual focus. As the potential of this, in a practical sense, deceptively simple ionisation technique is far from being fully developed, further exploration of the wider applicability of PADI and its strengths and limitations is ongoing. Various plasma parameters are systematically adapted to establish how this affects the analysis of a range of materials, including larger organic molecules and biomaterials. The mechanisms involved in generating PADI spectra form the second focus of our work. It has become apparent that temperature effects can have an important influence on the desorption of analyte molecules. How the different active species in the plasma affect the desorption in a more direct sense and how ions are formed is less well understood. Further investigation of this is essential to enable better analysis and interpretation of the, at times, complex spectra and to maximise the potential of PADI.
Conclusions
Surface analysis by mass spectrometry has experienced a step-change since the inception of ambient mass spectrometry removed the requirement for samples to be investigated under vacuum conditions. Approaches based on surface–plasma interactions are especially promising, including PADI. Whilst the mechanisms involved in generating PADI spectra still need to be unravelled, PADI shows significant promise to become a valuable and versatile tool in the instrumental arsenal available to the surface analyst.
Acknowledgements
The authors gratefully acknowledge support from Paul Davey and Ian Sinclair of AstraZeneca for ongoing research in the application of PADI for pharmaceutical analysis. David Barrett from the Centre for Analytical Bioscience of the School of Pharmacy at the University of Nottingham has kindly given access to the Thermo Exactive instrument used to record the data shown in Figure 4. Author JMSJ acknowledges the Iraqi Ministry of Higher Education and Scientific Research for financial support.
References
- J.C. Vickerman and D. Briggs (Eds), ToF-SIMS: Materials Analysis by Mass Spectrometry, 2nd Edn. IM Publications, Chichester, UK (2013). http://bit.ly/20CbI3a
- Z. Takáts, J.M. Wiseman, B. Gologan and R.G. Cooks, “Mass spectrometry sampling under ambient conditions with desorption electrospray ionization”, Science 306(5695), 471–473 (2004). doi: http://dx.doi.org/10.1126/science.1104404
- L.V. Ratcliffe, F.J.M. Rutten, D.A. Barrett, T. Whitmore, D. Seymour, C. Greenwood, Y. Aranda-Gonzalvo, S. Robinson and M.R.S. McCoustra, “Rapid surface analysis under ambient conditions using plasma-assisted desorption ionization (PADI) mass spectrometry”, Anal. Chem. 79, 6094–6101 (2007). doi: http://dx.doi.org/10.1021/ac070109q
- M.R.L. Paine, P.J. Barker and S.J. Blanksby, “Ambient ionisation mass spectrometry for the characterisation of polymers and polymer additives: a review”, Anal. Chim. Acta 808, 70–82 (2014). doi: http://dx.doi.org/10.1016/j.aca.2013.10.001
- M. Smoluch, P. Mielczarek and J. Silberring, “Plasma-based ambient ionization mass spectrometry in bioanalytical sciences”, Mass Spectrom. Rev., in press (2015). doi: http://dx.doi.org/10.1002/mas.21460
- T.L. Salter, F.M. Green, N. Faruqui and I.S. Gilmore, “Analysis of personal care products on model skin surfaces using DESI and PADI ambient mass spectrometry”, Analyst 136, 3274–3280 (2011). doi: http://dx.doi.org/10.1039/c1an15138j
- K. McKay, T.L. Salter, A. Bowfield, J.L. Walsh, I.S. Gilmore and J.W. Bradley, “Comparison of three plasma sources for ambient desorption/ionization mass spectrometry”, J. Am. Soc. Mass Spectrom. 25, 1528–1537 (2014). doi: http://dx.doi.org/10.1007/s13361-014-0924-x