Esterification is a chemical reaction involving an alcohol and a carboxylic acid (or an acid anhydride). Many esters have distinctive fruit-like flavours and they naturally occur in essential oils and plants. For example, ethyl isovalerate smells of apple, ethyl butyrate smells of pineapple and ethyl nonanoate smells of grape.
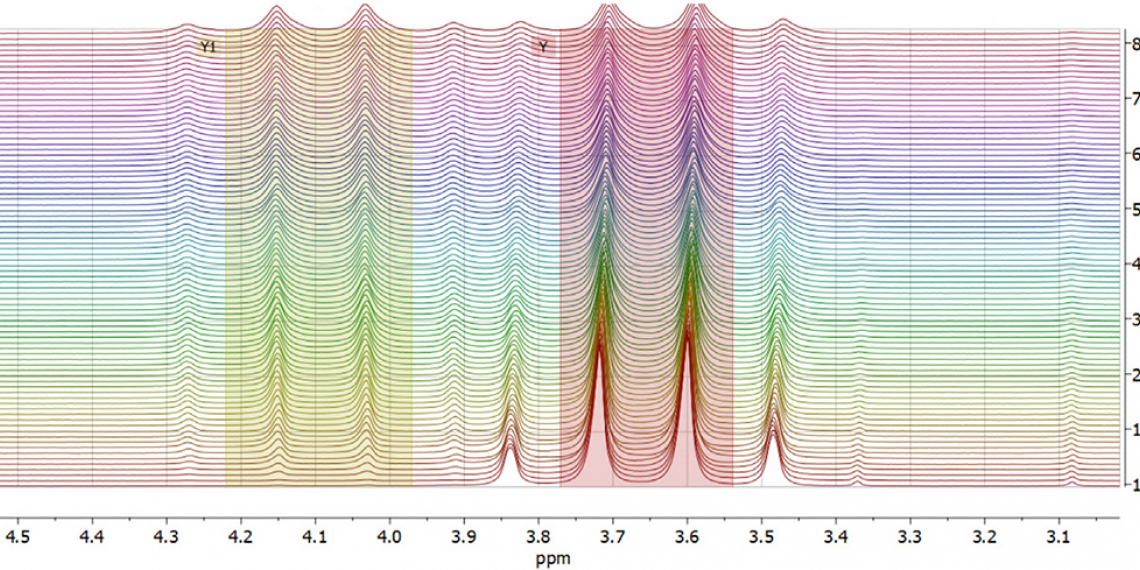
In this application note, an X-Pulse spectrometer equipped with a flow cell is utilised to monitor an esterification reaction. Ethyl ethanoate (also known as ethyl acetate) will be synthesised from ethanol and ethanoic acid (acetic acid) using an acid catalyst. The reaction proceeds through a series of equilibria and this means that unless the water that is produced is removed, the reaction will not go to completion. Ethyl ethanoate is important industrially as it is used as a solvent in enamels, lacquers and nail polish removers. Furthermore, ethyl ethanoate is used in the decaffeination process of tea and coffee. A flow setup will be used to monitor the formation of ethyl ethanoate over time and at different temperatures. Using this data, the activation parameters will be calculated using an Eyring–Polanyi plot.

