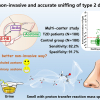
The Institut de recherches cliniques de Montréal (IRCM) in Canada and Nuclea Biotechnologies Inc. have formed a new strategic partnership to validate quantitative mass spectrometry-based assays for insulin, proinsulin and c-peptide. The goal of this partnership is to develop new assays to determine risk and progression of type 2 diabetes.
Benoit Coulombe, PhD, researcher at the IRCM, uses a translational proteomics platform, based on mass spectrometry, to develop assays ranging from biomarker discovery to validation. Nuclea focuses on diagnostic development and validation, with a recent emphasis placed on mass spectrometry-based assays.
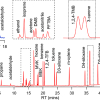
The partnership will include measuring clinical samples taken from patient cohorts and analysed in both locations (the IRCM and Nuclea) to demonstrate the precision, sensitivity and reproducibility of the assays. Nuclea will incorporate these new assays into its CLIA lab (clinical laboratory improvement amendments) in Cambridge.
“We are enthusiastic to partner with Nuclea on this project,” says Dr Coulombe, Director of the Translational Proteomics research unit and the Proteomics Discovery Platform at the IRCM. “Validation of these assays across the two laboratories will represent an added value in clinical and research environments.”
“Mass spectrometry-based assays for key protein targets have reached a tipping point in their development, and can now become routine in clinical laboratories,” says Mary Lopez, COO and VP of Proteomic Discovery at Nuclea Biotechnologies. “Nuclea’s high quality manufacturing and service capabilities combined with the new high-resolution mass spectrometry will provide urgently-needed tests for type 2 diabetes.”
Detecting and quantifying insulin and its therapeutic analogues could have applications in the medical or forensic fields, as well as in sports doping. New mass spectrometry-based assays have demonstrated a high degree of reliability and robustness, which are important factors for treatment decisions and to predict response to a given therapy in type 2 diabetes.
“The IRCM is pleased with this partnership, which recognises the importance of Dr Coulombe’s outstanding work in the field of biomarkers,” adds Tarik Möröy, PhD, IRCM President and Scientific Director. “The development of new biomarkers in the context of precision medicine is of great interest to the IRCM, as they are proving very useful for diagnosis and for the development of new drugs. Thanks to this technology, it will be possible to evaluate a patient’s response to treatment and, as a result, will lead to improved healthcare.”
“Nuclea has been working in the metabolic disease space for many years,” says Patrick Muraca, President and CEO of Nuclea Biotechnologies. “This partnership with the IRCM will provide Nuclea with inestimable expertise to offer a great deal of clinical value in the treatment and management of type 2 diabetes. This is an important area of focus in the US and Canada because 35% of US adults and 20 per cent of Canadian adults have metabolic syndrome.”