For more than 40 years, scientists have hypothesised the existence of enzyme clusters, or “metabolons,” in facilitating various processes within cells. Using gas cluster ion beam secondary ion mass spectrometry (GCIB-SIMS), researchers at Penn State have directly observed functional metabolons involved in generating purines, the most abundant cellular metabolites. The findings could lead to the development of novel therapeutic strategies that disrupt the progression of cancer.
“Our study suggests that enzymes are not haphazardly located throughout cells, but instead occur in discrete clusters, or metabolons, that carry out specific metabolic pathways”, said Stephen Benkovic, Evan Pugh University Professor and Eberly Chair in Chemistry. “Not only did we find proof that metabolons exist, but we also found that this metabolon occurs near mitochondria in cancer cells.” The findings were published in Science.
In the study, the team searched for a specific kind of metabolon, called a “purinosome,” that was thought to carry out de novo purine biosynthesis, the process by which new purines—building blocks of DNA and RNA—are synthesised. The researchers investigated these purinosomes within HeLa cells, a cervical cancer cell line commonly used in scientific research.
“We have shown that the de novo purine biosynthetic (DNPB) pathway is carried out by purinosomes consisting of at least nine enzymes acting together synergistically to increase their overall activity by at least by seven-fold”, said Vidhi Pareek, assistant research professor, Department of Chemistry and the Huck Institutes of Life Sciences.
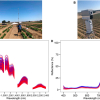
The researchers identified the purinosomes, which were less than a µm in diameter, using a novel imaging system developed by Nicholas Winograd, Evan Pugh University Professor of Chemistry, and colleagues. “The technique utilises gas cluster ion beam secondary ion mass spectrometry (GCIB-SIMS) to detect intact biomolecules with high sensitivity and allow in situ chemical imaging in single cells”, said Hua Tian, assistant research professor, Department of Chemistry and the Materials Research Institute. “This was vital for the study since we are dealing with very low concentration of molecules in individual cancer cells.”
Nicholas Winograd, Evan Pugh University Professor of Chemistry, has worked for 35 years to develop new techniques, including high-resolution GCIB-SIMS, that can provide sub-cellular chemical information. “Now, at the end of my career, I am finally seeing this imaging approach reveal the presence of purinosomes, and perhaps next, observe that a cancer drug actually makes it into a purinosome where it can be most effective”, he said.
Importantly, the team found that the DNPB pathway occurs in a channelled manner and the juxtaposition of purinosomes to the mitochondria facilitates uptake of substrates generated by the mitochondria for utilisation in the pathway. Channelling occurs when enzymes are located close together so that the molecules produced are quickly transferred and processed along the enzymatic pathway, restricting equilibration with the bulk cytosol.
“Our experiment allowed us to show that the efficiency of the de novo purine biosynthetic pathway is increased by channelling and that the proximity of purinosomes near mitochondria is consequential for the pathway”, said Benkovic. “These findings open the door to the study of a new class of cancer therapeutics; for example, the design of a molecule that can disrupt purinosomes’ juxtaposition with mitochondria.”