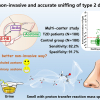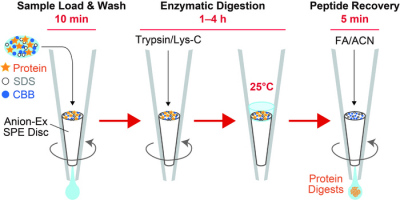
In recent years, proteomics research using mass spectrometry (MS) to comprehensively investigate the protein components of biological samples has become very popular. In order to detect proteins by MS, it is necessary to extract the protein components contained in the cells and digest them with proteases to a peptide size that is easy to analyse by MS. Protein digestion in this manner typically requires a long reaction time of 20 hours or more. Sodium dodecyl sulfate (SDS), a surfactant often used in protein extraction processes, interferes with MS and protein digestion, and must be thoroughly removed prior to analysis. However, that process comes with a cost, namely sample loss. To overcome these challenges, the researchers from Ehime University, Japan, developed an innovative sample preparation method, AnExSP (anion-exchange disc-assisted sequential sample preparation), which uses a µL-sized spin column fitted with anion-exchange solid-phase extraction discs in a pipette tip called a StageTip as a tool for protein digestion.
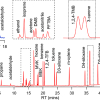
They succeeded in completing the enzymatic digestion process in a minimum of 60 min by enriching the protein components on the disc surface. Furthermore, they established optimal conditions for eluting only the digested peptides from the discs while keeping the SDS contained in the sample on the discs, and achieved sample pretreatment with simple operation and minimal loss. By combining AnExSP with data-independent acquisition (DIA) analysis by Orbitrap mass spectrometry, they succeeded in detecting approximately 7000 different protein components in 1 µg of human cultured cell protein extract. By establishing sample pretreatment conditions using the reduced-size StageTip in the future, AnExSP pretreatment can be used for single-cell proteomics research, which is of growing interest in recent years.
AnExSP can also be used for pretreatment of proteins separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE), which is commonly used in protein experiments. Compared to conventional pretreatment methods, AnExSP demonstrated superior performance, especially in the detection of long-chain digested peptides. In this study, they combined AnExSP and SDS-PAGE with a chemical cross-linking method for protein complexes, and succeeded in simple purification of target protein complexes and efficient detection of long-chain cross-linked peptides that are difficult to detect with conventional methods. Because of its far-reaching versatility, AnExSP is expected to be applied to highly sensitive structural analysis methods for trace protein complexes in biological samples.