Nuclear Magnetic Resonance (NMR) News
Bruker and their partner Diagnosticum are forming the Hungarian Wine Consortium to develop a new programme to authenticate and identify Hungarian wines.
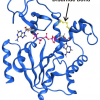
The structures and functions of a transient enzyme state have been mapped for the first time using NMR spectroscopy and X-ray crystallography.
Salmon is one of the most popular edible fish of all. Shops sell fish caught in the wild, but their main produce is salmon from breeding farms, the effluent from which can pollute rivers, lakes and oceans. German and Chilean scientists have used fluorescence measurements, high-resolution mass spectrometry and nuclear magnetic resonance spectroscopy to answer this question.
Lewis E. Kay is to receive a 2017 Canada Gairdner Award for his contributions to the field of biomolecular NMR spectroscopy.
numares AG and Oxford University are collaborating to develop an nuclear magnetic resonance (NMR)-based in vitro diagnostic (IVD) test to improve therapeutic decision making for patients with multiple sclerosis (MS). This is based on research conducted at Oxford to differentiate MS patients by metabolic biomarkers using NMR spectra. numares will provide its Magnetic Group Signaling® (MGS®) technology to Oxford to advance research toward the creation of a non-invasive diagnostic test.
NMR spectroscopy has shown a molecule self-assembling into different forms passing from solution state to solid state and back again.
A superconducting insert coil made from a copper-oxide-based ceramic, YBCO, has raised the magnetic field achievable to 25 Tesla.
New method allows hyperpolarised xenon gas, to be dissolved into minute samples of substances without disrupting their molecular order during NMR spectroscopy.
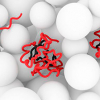
The protein α-synuclein plays an important role in Parkinson’s and other neurodegenerative diseases. Although a considerable amount is known about the structure of the protein within the Parkinson’s-typical amyloid deposits, nothing was known about its original state in the healthy cell up to now. High-resolution nuclear magnetic resonance and electron paramagnetic resonance spectroscopy have helped to visualise the protein in healthy cells.
Searching for the precise, complexly folded three-dimensional structure of a protein can be a long, intensive process with uncertain direction. A technique based on nuclear magnetic resonance spectroscopy offers a solution.
Bruker has announced five orders for ultra-high field (UHF) nuclear magnetic resonance (NMR) spectroscopy systems from Europe and Brazil in recent months. These UHF systems have been funded for cutting-edge NMR research in structural biology, intrinsically disordered proteins (IDPs), membrane proteins, macro-molecular complexes and interactions, cell biology, disease research, as well as in advanced materials research. Bruker defines UHF as NMR systems with 1H proton frequency of 900 MHz or above.
Magritek’s Spinsolve Benchtop NMR spectrometer is being used in the Chemistry Department of the University of Queensland as part of the undergraduate laboratory curricula.
Understanding the extremely fast atomic mechanisms at work when a protein transitions from one shape to another has been an elusive scientific goal for years, but an essential one for elucidating the full range of protein function. How do proteins transition between distinct shapes without unfolding in the process? Until now, this question has been a hypothetical one, approached by computation only rather than experimentation.
Jeol have introduced the latest version of their Delta NMR software, which is used to power their ECX and ECA series NMR systems.