Dirk W. Lachenmeier, Marina Gary, Yulia B. Monakhova, Thomas Kuballa and Gerd Mildau
Chemisches und Veterinäruntersuchungsamt (CVUA) Karlsruhe, Weissenburger Strasse 3, D-76187 Karlsruhe, Germany. E-mail: [email protected]
Introduction
In the context of the quality control of both vegetable oils and fats, and oil-containing cosmetics, lipid stability is an important parameter. It not only impacts the shelf life of products, but also their safety, since some oxidation products (e.g. malonaldehyde, cholesterol oxides) have toxic properties.1 The term rancidity refers to the off odours and flavours resulting from lipid oxidation (also called autoxidation) as the predominant mechanism.1,2 The oxidation occurs via a self-sustaining free radical mechanism that produces hydroperoxides (initial or primary products) that undergo scission to form various secondary products, including aldehydes, ketones, organic acids and hydrocarbons (final or secondary products).1 The autoxidation is promoted by heat, light, certain metals (iron and copper) and lipoxygenases.2
A classical wet-chemical test to detect oxidative fat deterioration is the peroxide value determined by redox titrimetry. The test is still widely applied in fat chemistry, but lacks a clear relationship with aroma defects. For this reason, headspace gas chromatographic (GC) measurement of individual carbonyl compounds is often preferred, with hexanal typically used as the indicator compound.1,3
Nuclear magnetic resonance (NMR) spectroscopy has been proposed as probably the best technique for screening food extracts.4 In a comparison with spectroscopic screening techniques, much more significant information was provided using NMR spectroscopy as compared with near infrared or Fourier transform infrared spectroscopy, and selective and sensitive qualitative and quantitative information could be gathered from NMR spectra.5 Even though 400 MHz NMR machines are still expensive, compared with many other analytical systems, the cost per sample for NMR can be very low, depending on the turnover achieved.6 Previous NMR research into the oxidative stability of lipids was generally focused on fatty acids and their changes.7 We are the first to use the aldehyde proton absorptions in NMR spectroscopy for quality control of oils, and in this article we discuss this development. As our institute not only investigates foods but also other consumer products such as cosmetics, we are sometimes confronted with “rancid” tasting lipsticks. This article therefore additionally discusses whether 1H NMR is suitable to confirm oxidative fat deterioration in cosmetics.
Sample preparation and instrumentation
200 mg of a liquid sample (plant oil) is diluted in 0.8 mL CDCl3, 0.6 mL of this mixture is used to fill an NMR tube and measured directly. 200 mg of solid samples such as lipsticks are dissolved in 1.4 mL CDCl3, but to facilitate their dissolution, the samples are warmed for 10 min at 50°C and then carefully shaken till homogenisation is reached. Following this, hydrophilic compounds are separated by extraction with 1 mL of water. After centrifugation, the CDCl3 phase is membrane-filtrated and 0.6 mL of the filtrate is poured into an NMR tube and directly measured. Each tube contains 0.3 mg tetramethylsilane as internal standard. For quantification, an external calibration with nonanal solutions (8–830 µg mL–1) is also undertaken.
All NMR measurements were performed on a Bruker Avance 400 Ultrashield spectrometer (Bruker Biospin, Rheinstetten, Germany) equipped with a 5-mm SEI probe with Z-gradient coils, using a Bruker Automatic Sample Changer (B-ACS 60). All spectra were acquired at 300.0 K. 1H spectra were acquired using Bruker standard 1D zg30 pulse sequence with 128 scans and two prior dummy scans. The sweep width was 20.5 ppm and the time domain of the FID, 132 kB. The data were acquired automatically under the control of ICON-NMR (Bruker Biospin, Rheinstetten, Germany), requiring about 25 min per sample.
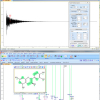
Aldehydes are quite distinctive in the NMR and have peaks in the 9–10 ppm region. For our screening purposes, we measure the total free aldehydes, defined as all compounds with an aldehyde group that show a proton signal similar to the one of nonanal (9.76 ppm) (among them are, for example, hexanal or the fragrance compound 7-hydroxy-citronellal). Typical 1H NMR spectra of aldehydes are shown in Figure 1.

Method validation
The lowest calibrant at 8 µg still shows an NMR signal which is more than 10× greater than the standard deviation of the noise. This was arbitrarily set as the detection limit. For oil samples with a weight of 200 mg and a solvent volume of 0.8 mL, this approximates to about 40 mg kg–1 of sample. We have not further investigated the lower detection limit, as positive (i.e. rancid) samples show considerably higher levels of aldehydes, so that no optimisation for analysing trace amounts is necessary in this case. Validation of the method was further conducted by repeated sample preparation of differently spiked samples as well as of authentic rancid-tasting samples. The results are shown in Table 1. For the oil samples, the relative standard deviations (RSD) were always below 5%. For the cosmetics, the RSDs were slightly higher but still acceptably below 10%. The higher measuring errors for cosmetics can be explained by inhomogeneities in the samples’ matrix as we just scraped small pieces off the surface of the lipsticks. We did not homogenise a larger amount of sample material in order to assess the safety of the product judging only the aldehyde concentration of the outer lipstick part, which is that intended to be used by the consumer.
Table 1. Validation results for the determination of total aldehydes using 1H NMR spectroscopy.
Samples | Mean | Standard deviation | Relative standard deviation | Recovery |
Standard solution (n = 10) | 365.2 | 2.2 | 0.6 | 101.2 |
Spiked olive oil (n = 5) | 56.1 | 1.6 | 2.9 | 97.3 |
Authentic rancid grape seed oil (n = 10) | 103.6 | 3.3 | 3.2 | — |
Spiked lipstick (n = 5) | 245.0 | 18.9 | 7.7 | 98.1 |
Authentic lipstick (n = 5) | 65.8 | 4.1 | 6.2 | — |
Authentic rancid lipstick (n = 5) | 2256 | 137 | 6.1 | — |
Measurement of authentic samples
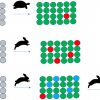
The developed methodology was used to analyse 72 oil and 38 cosmetic samples, which were submitted to our institute by governmental authorities in order to check their compliance with the food and cosmetics provisions. The results of our investigations are presented in Figure 2.

From the vegetable oils, 22 samples (31%) contained detectable levels of aldehydes, but only two contained levels above 100 mg kg–1, which also were characterised by a rancid taste. Most samples had a typical taste during organoleptical testing, which was confirmed by the very low or a not detectable content of aldehydes. In general, this non-representative survey of the German market shows a high product quality regarding oxidative stability. There appears to be no problem with over-storage and we also do not see a health risk for the population from the toxic oxidation products mentioned above.
From the analysed 38 lipsticks, 26 contained aldehydes above the detection limit (68%), and 15 had levels above 100 mg kg–1. The sample, which was most striking, however, was a lipstick characterised by a rancid taste and over 2000 mg kg–1 of total aldehydes (see Figure 3). Our results confirm the previous opinion that lipsticks are comparably problematic in regard to oxidative stability, depending on their formulation. During production, relatively high temperatures (>75°C) have to be used and pro-oxidants such as iron oxides may be contained as pigments.8 Additionally, unsaturated natural vegetable oils such as almond oil may bring considerable shelf- and brand-appeal,9 but may break down more quickly than the traditionally used castor oil as a major lipstick ingredient.8 Finally, less than optimal storage conditions and the frequent opening and closing during use may promote oxidation. It is therefore demanding to formulate oxidation-stable lipsticks, and we think that our method could also be used in an industrial setting for product development or accelerated shelf-life testings.

Conclusion
We conclude from this investigation that the routine application of NMR in the screening for oxidative stability is a useful addition to the standard range of analytical methods. It can be used without modifications for fatty foods as well as oil-containing cosmetics. The approach is advantageous as it minimises manual handling (i.e. only a very simple sample preparation has to be conducted) and allows one to automatically analyse a large number of samples (>50 per day). In case of positive results, the NMR results should be complemented by the more specific GC analysis, which allows for the differentiation of aldehydes that are not separated by NMR spectroscopy.
Acknowledgements
The authors are grateful to Jürgen Geisser for excellent technical assistance. Yulia B. Monakhova was supported by a combined DAAD (German Academic Exchange Service) and Russian Ministry of Education grant (No. 2.2.2.3/9033).
References
- S.F. O’Keefe and O.A. Pike, in Food Analysis, 4th Edn, Ed by S.S. Nielsen. Springer, New York, pp. 239–260 (2010).
- V.A. Vaclavik and E.W. Christian, Essentials of Food Science. Springer, New York, pp. 273–309 (2008).
- H.D. Belitz, W. Grosch and P. Schieberle, Food Chemistry. Springer-Verlag, Berlin, pp. 640–669 (2009).
- G. Le Gall and I.J. Colquhoun, in Food Authenticity and Traceability, Ed by M. Lees. Woodhead Publishing Ltd, Cambridge, UK, pp. 131–155 (2003).
- D.W. Lachenmeier, Food Chem. 101, 825–832 (2007).
- D.W. Lachenmeier, E. Humpfer, F. Fang, B. Schütz, P. Dvortsak, C. Sproll and M. Spraul, J. Agric. Food Chem. 57, 7194–7199 (2009).
- D.W. Lachenmeier, E. Humpfer, F. Fang, B. Schütz, P. Dvortsak, C. Sproll and M. Spraul, J. Agric. Food Chem. 57, 7194–7199 (2009).
- S.P.J.N. Senanayake and F. Shahidi, J. Food Lipids 14, 217–231 (2007).
- R. Kleiman, M. Cummings and J. Reinhardt, Soap Cosmet. 76, 31–55 (2000).
- T. Oldfield and T. Carter, SOFW J. 131, 38–41 (2005).