Kareem Elsayada, and Francesca Palombob,
and Francesca Palombob,
aVBCF – Advanced Microscopy, Dr. Bohr-Gasse 3, Vienna, A-1030, Austria. E-mail: [email protected]
bSchool of Physics and Astronomy, University of Exeter, Stocker Road, Exeter EX4 4QL, UK. E-mail: [email protected]
When confronted with the phrase “optical spectroscopy” either fluorescence spectroscopy, Raman spectroscopy or maybe infrared absorption spectroscopy, will come to mind. These well-established techniques, which can now readily also be performed with commercial hand-held devices, have found a broad and continuously expanding set of applications that span quality control, medical diagnostics and even art forensics. Raman spectroscopy generally measures resonances at frequency shifts in the range 500–4000 cm–1 (15–120 THz) relative to the excitation wavelength, and can yield detailed information on the molecular composition of a sample. But what happens when one goes to smaller spectral shifts?
As one ventures below the Raman fingerprint region (<500 cm–1) there appears not to be much exciting beyond a smooth background for a while, until suddenly in the region of ~1–100 cm–1 (0.03–3 THz) one sees some bright structured peaks again. This is the so-called THz, or low-frequency Raman, spectral region where inelastic scattering arises from coupled molecular bond vibrations. These vibrations are slower (lower energy) than the individual bond vibrations, and though still confined to a given molecule, are a little more extended across different parts of the molecule. They can yield useful structural information and have over the last decade received much interest from among others the pharmaceutical industry for quality control applications. But what if one moves even closer to the blinding excitation laser frequency?
As we venture below the THz–Raman spectral region, again for a while one appears to only find a largely feature-less spectral landscape that varies little from sample to sample. Looking onwards to even lower energies, more elaborate spectrometers are soon required, which utilise either passing a beam numerous times between scanning cavities1 or using special high-angular dispersive optical elements.2 Dangerously close to the excitation laser frequency at around 0.3 cm–1 (10 GHz frequency) one, however, now sees a single or sometimes two or three sharp peaks again. One would be forgiven for thinking this is the result of a laser instability, but it is not (in this case). This relatively poorly explored area (as far as life-science and biomedical applications go) is the territory of Brillouin light scattering spectroscopy. The peaks one observes here are due to the scattering from so-called acoustic phonons, or low-energy collective molecular vibrations that span hundreds to thousands of atoms.3 These “modes”, which are ever present at finite temperatures, will depend on the structure of the sample and are determined by an effective collective response (as opposed to the properties of a single molecule as is the case in the higher energy excitations). Typically travelling at a few thousands of metres per second, they have an effective wavelength on the order of ~100 nm and a relatively long lifetime, decaying over a distance of up to several microns. Seen semi-classically, the observed peak(s) in the spectrum can be understood to be the inelastic scattering of light from a density wave (or moving Bragg grating), with the observed frequency shift directly proportional to the velocity of the wave, viz. the Doppler shift. The velocity in turn is related to different components of the stiffness tensor (the precise components depending on the measurement geometry), and thus can be used to calculate elastic moduli (via the Christoffel equation). To this end it has been utilised for almost half a century in geology as well as condensed matter physics.
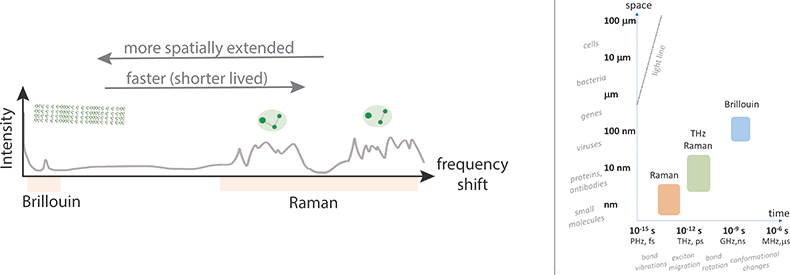
Figure 1. Sketch of combined Brillouin and Raman scattering spectra (left), and characteristic spatial and temporal scales associated with the underlying processes in relation to the sizes of different structures and time-scales of different processes (right).
Over the last decade there has been renewed interest in using Brillouin spectroscopy to study biological specimens. This to a large extent has been driven by the realisation of a novel imaging spectrometer design, based on so-called Virtually Imaged Phase Arrays (VIPAs),2 which allow for fast spectral measurements at laser powers conducive to studying live biological samples. Together with a confocal detection scheme, the prospect of optically measuring the elastic modulus with diffraction limited resolution and then mapping this in 3D in a live sample sounds very appealing, both for life-science research (mechanobiology), but also for potential medical diagnostic applications. There are, however, several caveats, and care has to be taken when interpreting the Brillouin derived elastic moduli. First, unlike the Young’s Modulus measured with Atomic Force Microscopy (AFM) and other stress–strain techniques, or the Shear Modulus measured in microrheology, the elastic modulus measured in Brillouin scattering in the most commonly used backscattering geometry is the so-called Longitudinal Modulus, which also depends on the compressibility of a sample. (N.B. Longitudinal modulus derives its name from the longitudinal modes it measures, which propagate along the same direction as the incident and detected radiation.) As such, the value for incompressible materials such as liquids will be very large and may in certain cases be dominant. In addition, due to the high frequency (GHz) of the probed acoustic phonons, one is effectively probing mechanical properties also in what can be considered the high-frequency regime, where distinct mechanical relaxation rates are at play to those at lower frequencies more commonly probed with other techniques. Finally, to derive the elastic modulus from the frequency shift of the Brillouin peak requires knowledge of the ratio ρ/n2 (where ρ is the mass density and n the refractive index of the material)—which is not always known or readily accessible in a sample, particularly in heterogeneous media, and requires parallel measurements. To a first approximation, it may be assumed that the factor ρ/n2 is constant (Lorentz–Lorenz relation), however, the validity of this assumption may not always be justifiable. In light of all of this, extra care has to be taken both to correctly interpret the Brillouin-derived elastic moduli and when drawing any correlations with parameters obtained from other techniques.
Despite these challenges, Brillouin microspectroscopy has, over the last decade, been applied in studying various systems of biomedical interest in order to assess potential diagnostic capability. A motivation for doing so is that mechanical damage in tissue can be associated with numerous pathological states such as osteoarthritis and keratoconus, and the ability to probe these changes on a sub-micron scale using a contactless all-optical technique would prove beneficial in clinical diagnostics. Contact techniques on the other hand would present the disadvantage of decoupling engineering strain from the real strain applied to the material. But since Brillouin microscopy is contactless and probes spontaneous acoustic modes that are intrinsic to the material, cells and tissues can be analysed in their true native environment and without any mechanical perturbations. Much effort is also being devoted to making the technique more viable for optical diagnostics, such as combining with endoscopy, in a similar way as has been done with other vibrational spectroscopy techniques.
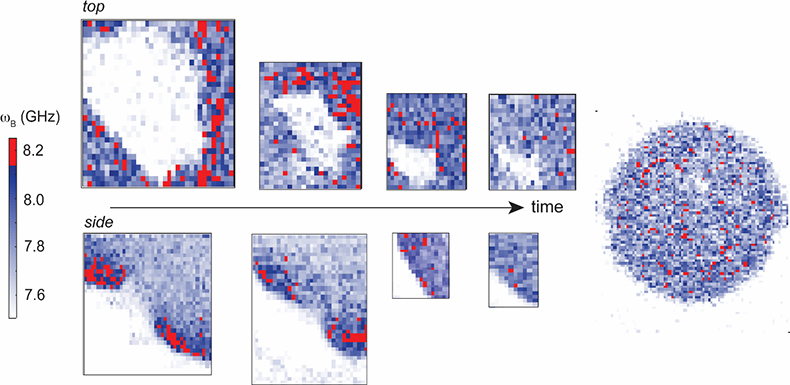
Figure 2. Time-series Brillouin frequency shift (ωB) spatial maps of a live developing sea anemone (Nematostella vectensis) showing increased Brillouin shift (related to stiffness) around the invagination area at the beginning of embryogenesis (pixel size = 1 µm). Acquired using a confocal microscope attached to a dual-stage cross-dispersion VIPA spectrometer (1.3 NA objective lens, 532 nm laser power ~1.5 mW at sample with 120 ms dwell time per pixel). Sample courtesy of E. Puhlyakova and U. Technau (University of Vienna).
Studying samples of biological and biomedical interest, however, does not come without its own associated challenges. Biological samples are generally very complex structures with heterogeneities in composition, optical and mechanical properties on a microscopic scale, making it more challenging to extract quantitative information. Within the approximation of a homogeneous ratio ρ/n2, which is plausible far away from electronic resonances, the Brillouin shift can, however, be expected to scale directly with the elasticity or stiffness of a material, such that large Brillouin frequency shifts can be assumed to correlate with increased rigidity and vice versa. In this regard, Brillouin microspectroscopy can provide a novel contrast mechanism for mapping micromechanics also within cells and tissues. Hydration is also known to have a prominent effect on the measured properties, and thus needs to be carefully controlled, together with sample preparation in general. To date, the potential biological and medical applications of Brillouin microspectroscopy have been demonstrated on a variety of cells and tissue types. These range from ex vivo studies on histological sections from tissue biopsies,4,5 to in vitro applications in live cells6 as well as live tissue7 and organisms.8 It has also been applied in the analysis of liquid biopsies (e.g. Reference 9). In regards to biomedical applications, it is probably most advanced in the field of ophthalmology—for keratoconus assessment, where it is currently undergoing clinical trials.10 Parallel efforts have been devoted to instrument development to overcome limitations in acquisition time and to achieve high contrast for the analysis of more opaque materials.
Though much progress has been made over the last few years in terms of both instrument development and applications, there are at this point still more questions than answers, such as: How do we best understand these low energy modes probed using Brillouin scattering in complex heterogeneous biological system? What biological/biophysical relevance could their properties and the derived high-frequency elastic moduli have?
One thing that is for sure is that the Brillouin measured elastic modulus clearly varies between regions of a biological sample, even if sometimes only by small amounts. Exactly what this is telling us and for what it is most useful or relevant is currently an area of active debate and remains to be seen.
References
- J.R. Sandercock, “Brillouin scattering study of SbSI using a double-passed, stabilised scanning interferometer”, Opt. Commun. 2, 73–76 (1970). doi: https://doi.org/10.1016/0030-4018(70)90047-7
- G. Scarcelli and S.-H. Yun, “Confocal Brillouin microscopy for three-dimensional mechanical imaging”, Nat. Phot. 2, 39–43 (2008). doi: https://doi.org/10.1038/nphoton.2007.250
- B.J. Berne and R. Pecora, Dynamic Light Scattering: With Applications to Chemistry, Biology, and Physics. Dover Publications, New York (2000).
- F. Palombo, M. Madami, N. Stone and D. Fioretto, “Mechanical mapping with chemical specificity by confocal Brillouin and Raman microscopy”, Analyst 139, 729–733 (2014). doi: https://doi.org/10.1039/C3AN02168H
- G. Antonacci, R.M. Pedrigi, A. Kondiboyina, V.V. Mehta, R. de Silva, C. Paterson, R. Krams and P. Török, “Quantification of plaque stiffness by Brillouin microscopy in experimental thin cap fibroatheroma”, J. Roy. Soc. Interface 12, 20150843 (2015). doi: https://doi.org/10.1098/rsif.2015.0843
- G. Scarcelli, W.J. Polacheck, H.T. Nia, K. Patel, A.J. Grodzinsky, R.D. Kamm and S.H. Yun, “Noncontact three-dimensional mapping of intracellular hydromechanical properties by Brillouin microscopy”, Nat. Meth. 12, 1132–1136 (2015). doi: https://doi.org/10.1038/nmeth.3616
- K. Elsayad et al., “Mapping the subcellular mechanical properties of live cells in tissues with fluorescence emission–Brillouin imaging”, Science Sig. 9(435), rs5 (2016). doi: https://doi.org/10.1126/scisignal.aaf6326
- R. Schüßler et al., “Mechanical mapping of spinal cord growth and repair in living zebrafish larvae by Brillouin imaging”, Biophys. J. 115, 911–923 (2018). doi: https://doi.org/10.1016/j.bpj.2018.07.027
- Z. Steelman, Z. Meng, A.J. Traverso and V.V. Yakovlev, “Brillouin spectroscopy as a new method of screening for increased CSF total protein during bacterial meningitis”, J. Biophot. 8, 408–414 (2015). doi: https://doi.org/10.1002/jbio.201400047
- A. Yun, Study to Test the Diagnostic Potential of Brillouin Microscopy for Corneal Ectasia. ClinicalTrials.gov Identifier: NCT02118922) (2014). https://clinicaltrials.gov/ct2/show/NCT02118922?term=NCT02118922&rank=1


